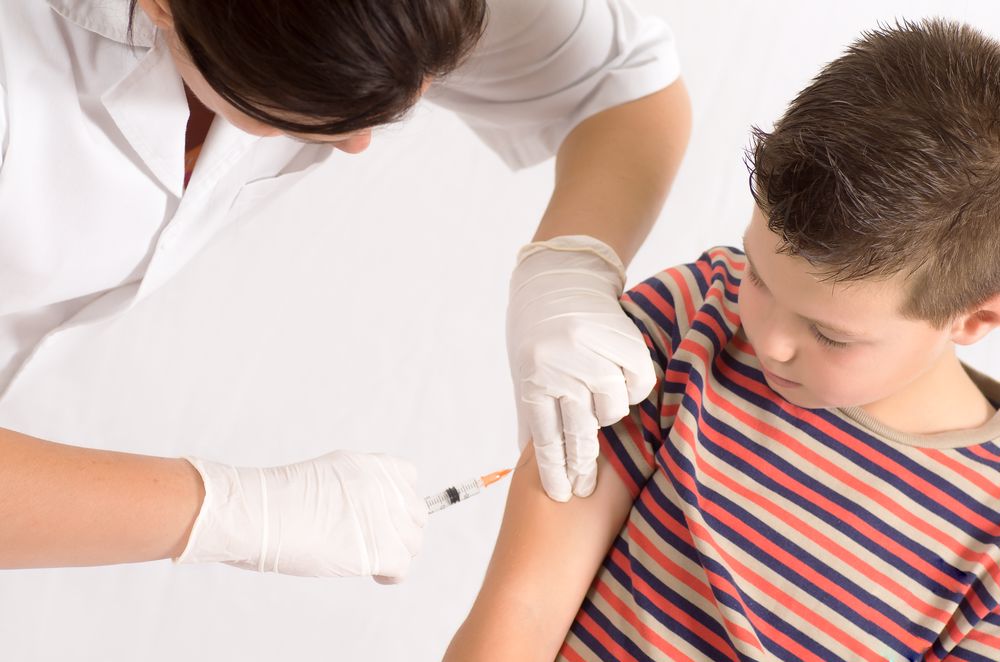
In 2020, the COVID-19 pandemic spread throughout the world, and everyone’s way of life changed. Due to the contagious nature of the disease, it’s highly dangerous, and people have had to prioritize safety and social distancing to slow the spread. Thankfully, there is an end in sight: the COVID-19 vaccine.
During a worldwide crisis, countries need to come together. Everyone needs equal access to the vaccine if we are to ever beat the pandemic, and that requires a coordinated effort to distribute vaccines quickly, safely and on a global scale.
But how is that possible?
COVAX: What is it?
COVID-19 Vaccines Global Access (COVAX) is a worldwide initiative organized by the World Health Organisation, among other entities. It’s aimed at ensuring equal access to COVID-19 vaccines across the world.
Through this initiative, vaccine manufacturers will provide safe and efficient vaccine rollout to participating countries, regardless of their involvement in the development of the vaccine. This process will ensure that vaccinations occur as quickly as possible on a global scale, slowing down the spread of the virus if not halting it entirely.
As of August 2020, there are nine candidate vaccines available through the initiative, including the most commonly known Moderna vaccine and Pfizer vaccine, with another nine still under review. With several different vaccine manufacturers supplying the drug to countries in need, distribution can happen more quickly.
Experts at the forefront of this initiative agree that this is not a time for competition.
‘COVID-19 is an unprecedented global health challenge that can only be met with unprecedented cooperation between governments, researchers, manufacturers and multilateral partners,’ said Dr Tedros Adhanom Ghebreyesus, Director-General of the World Health Organisation.
However, COVAX alone will not ensure successful vaccine distribution throughout the world. Comprehensive logistics, adequate cold chain management and high-end technology are essential pieces of the puzzle.
Next steps
The vaccine is made of raw materials that are temperature sensitive. Therefore, the vaccine vials must be stored at appropriate temperatures at all times. For the COVID-19 vaccine, that temperature range is 2-8 degrees Celsius, according to the Australian Government Department of Health National Vaccine Storage Guidelines.
The vaccines cannot be in an environment outside of the appropriate climate for more than 15 minutes. This means throughout every step of the cold chain — the journey from the laboratory all the way to the health centre where the vaccine is administered — the vials must be refrigerated, and the internal temperature of the storage units must be constantly monitored.
This requires specialized equipment and maintenance every step of the way.
‘COVID-19 is an unprecedented global health challenge that can only be met with unprecedented cooperation between governments, researchers, manufacturers and multilateral partners.’
Vaccine storage
If the environment in which the vaccine is stored reaches a temperature outside of the acceptable range, the occurrence is known as a cold chain breach, and it could damage or destroy the drug.
In order to ensure that the vaccine storage unit consistently remains at the appropriate temperature, professionals at every step of the cold chain must utilise adequate temperature monitoring technology. That’s where Testo comes in.
We have a range of products for every step of the cold chain. The cold chain begins as soon as the vaccine is developed, so the storage units at the laboratories need to be monitored for temperature changes. Our measurement technology for cleanrooms comes with high-precision probes for intuitive temperature measurement, plus optional Bluetooth capability for easy temperature recording and data storage.
Additionally, the refrigerators and freezers need to be monitored both in the laboratory and the health centre. The testo Saveris 2 monitors temperature and humidity changes, both of which impact the safety and efficacy of vaccines. Also WiFi and Bluetooth capable, this temperature logger effortlessly records changing temperatures and stores all data to the Testo Cloud. The system is customisable, making it ideal for new drugs like the COVID-19 vaccine.
Vaccine transportation
The consistent movement of the vaccine from one environment to another makes distribution especially difficult. With these changes often come temperature fluctuations. Therefore, professionals need to be mindful of the movements that have an effect on the temperature of the storage units as well as the functionality of the units in general.
This is where the testo 184 data loggers come in. These temperature monitoring devices are ideal for transportation purposes because their function is uninterrupted, even during item transfers or unique events. They are compact and easy to handle, and they record and store a large amount of temperature data. These easily transportable devices are the key to ensuring smooth transportation with few to no cold chain breaches, which is essential in safe, fast vaccine rollout throughout the world.
Vaccine monitoring
Once the vaccines are administered, the work is still not over. The Therapeutic Goods Administration (TGA) released a COVID-19 Vaccine Safety Monitoring Plan to ensure updated information on vaccine efficacy, safety and risks.
This plan relies on vaccine recipients informing the local health department of severe side effects. This data will not only lead to new discoveries about groups who are at higher risk for vaccine side effects than others, but it will also help professionals spot errors within the cold chain or administration logistics.
In order for this data to be useful, professionals must also have readily available, accurate data about the temperatures that the vaccines experienced at every step of the cold chain. This will eliminate the possibility of malfunctioning technology or cold chain breaches and lead to more research on vaccine risks.
Vaccine logistics and cold chain management rely heavily on the right technology. Testo’s robust, high-end solutions are the answer.
Contact us today for more information.







 Reduce cooking oil costs while ensuring quality
Reduce cooking oil costs while ensuring quality Expert knowledge on CO2 monitoring
Expert knowledge on CO2 monitoring Refrigeration knowledge - in 3 modules
Refrigeration knowledge - in 3 modules