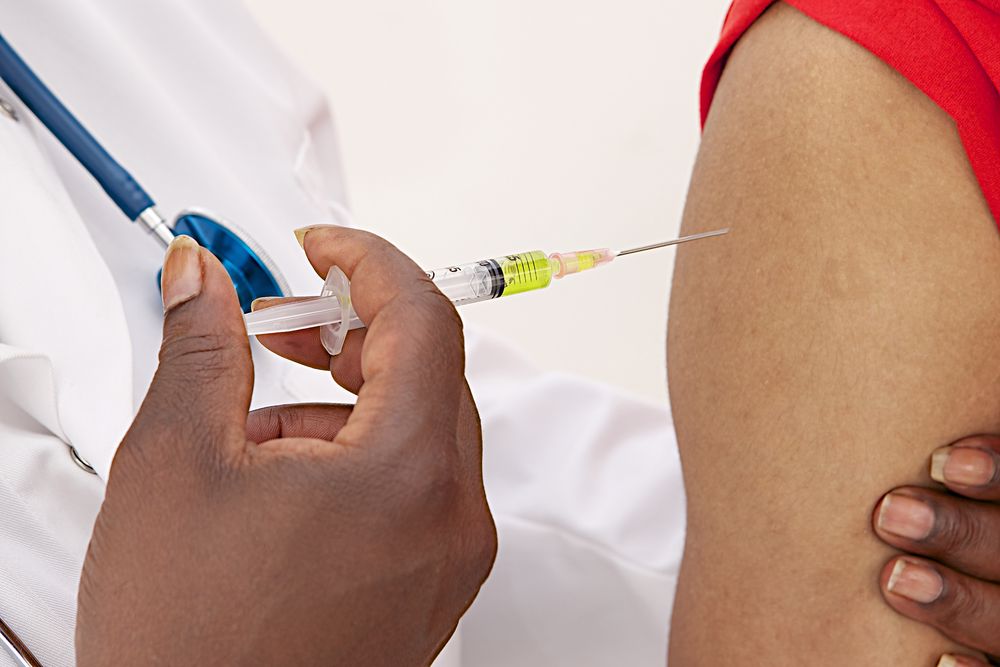
In 2020, the COVID-19 virus became a worldwide pandemic, making social distancing a new way of life all around the world. Millions of people lost loved ones, and some countries are still in the midst of an emergency. For many communities, there is only one thing they can talk about: the vaccine.
There have been pandemics before this, many of which required enhanced precautions, social distancing and, ultimately, a new drug. Developing a drug in the midst of a worldwide crisis is only half the battle, however — the other half is storage, distribution and administration.
Who sets vaccine storage guidelines, and why?
The Australian Department of Health set national vaccine storage guidelines, which were most recently updated in April 2020.
These guidelines are designed to ensure that vaccines are kept in safe conditions. This will lengthen their shelf life and allow for less vaccine loss, which leads to faster widespread distribution.
Vaccines contain ingredients that are sensitive to their environments, especially temperature. Storing a vaccine in the wrong climate can render the drug ineffective, sometimes alarmingly quickly.
Therefore, immunisation providers need adequate vaccine storage management and consistent temperature monitoring for efficient rollouts.
How should vaccines be stored?
According to the Department of Health, vaccines should be stored between 2-8 degrees Celsius. In addition to this regulation, facilities carrying the COVID-19 Pfizer vaccine must manage and monitor temperatures of -80 Celsius.
Temperature regulation should begin the minute that the vaccine is produced and should continue until it is administered. The process of storing the drug at the appropriate temperature throughout every step of distribution is known as the cold chain.
If the temperature of the vaccine storage unit deviates from the acceptable range, a cold chain breach occurs. When this happens for a period of time greater than 15 minutes, cold chain breach protocol must be implemented.Cold chain breach protocol
According to the Department of Health, to rectify the situation after a cold chain breach:
- Isolate affected vaccines and prepare to transfer them into a temporarily monitored vaccine storage unit, using ice packs or gel packs.
- Keep vaccines stored between 2-8 degrees Celsius, but label them ‘Do Not Use’ until they are ready to be transferred.
- Contact your state or territory health department to inform them of the cold chain breach.
- Continue storing vaccines unless directed to discard them by the department of health.
- Evaluate the storage unit in which the cold chain breach occurred to identify the cause, and remedy the problem.
Vaccines face heightened risk of cold chain breach during transportation due to the frequent changes in storage environment.
 Developing the COVID-19 vaccine is only half the battle. It must be stored and transported at appropriate temperatures to maintain vaccine safety and efficacy.
Developing the COVID-19 vaccine is only half the battle. It must be stored and transported at appropriate temperatures to maintain vaccine safety and efficacy.How can vaccines be transported?
Since vaccines need to be stored at a consistent, cold temperature, they must be kept in portable purpose-built vaccine refrigerators during transportation.
These refrigerators are specifically designed for vaccine storage. The cabin temperature is controlled to stay within an acceptable temperature range for the vaccine being transported, and if there is an issue that causes a cold chain breach, the system sets off an alarm so the distributor can remedy the problem within the 15-minute window.
Purpose-built vaccine refrigerators also come with built-in data loggers to consistently monitor temperature changes. Plus, they have the capability to stabilise the storage temperature after the unit has been opened.
In order to distribute vaccines throughout the world and keep them stored at the required temperature while waiting for administration, immunisation providers must utilise purpose-built vaccine refrigerators — both portable and stationary.
Once the vaccines reach the destination, there is still work to be done to prevent wastage at points of care. On-site temperature monitoring must be conducted at vaccine administration locations, plus, immunisation providers must schedule vaccinations efficiently so as many doses as possible are used. Even in cases of safe vaccine storage, drugs only last a few days after arrival at the site, and wasting them because of scheduling errors can slow the vaccine rollout.
Testo’s solutions
To control vaccine storage conditions, temperatures must be consistently monitored. To be sure that the storage climate is safe, temperature measuring tools must be reliable and efficient. During times of crisis, like the COVID-19 pandemic, there is little room for error or delays.
That is why Testo’s temperature monitoring devices are the solution. Our products, which include environmental and temperature monitoring in both stationary and mobile freezers, are capable of automated continuous monitoring and recording of temperature data. They take high precision measurements, ensuring that efficacy of drugs will not be compromised due to an inaccurate temperature reading. Our cutting-edge technology is the safest solution for a process, like vaccine storage, that requires constant attention and care.
We have a range of products for a variety of situations. The testo Saveris 2 T3 is a WiFi-capable data logger system, so it can be installed and operated through an internet browser. It is versatile, user-friendly and, most importantly, reliable. It’s the ideal solution for storage temperature monitoring in stationary freezers.
For transportation purposes, the testo 184 is a data logger that monitors temperature changes throughout the entire cold chain. It’s easy to operate, which is essential for a product that will be handed off to a new person once it reaches a destination. The testo 184 allows for uninterrupted temperature monitoring to ensure few to no cold chain breaches.
While enhanced safety measures, social distancing and improved hygiene can be effective temporary solutions, effective vaccine rollouts are what will hopefully put an end to the COVID-19 pandemic. This can only be possible with proper storage and transportation equipment. That’s where Testo comes in.
For more information, contact us today.







 Reduce cooking oil costs while ensuring quality
Reduce cooking oil costs while ensuring quality Expert knowledge on CO2 monitoring
Expert knowledge on CO2 monitoring Refrigeration knowledge - in 3 modules
Refrigeration knowledge - in 3 modules