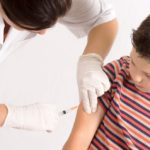
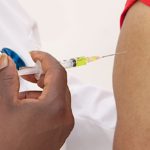
Developing the COVID-19 vaccine required many months of clinical trials, and scientists are now confident the vaccines available are safe and effective. However, the work is far from over.
Now that vaccines are being distributed and administered across Australia, it’s important that these processes undergo active surveillance and safety monitoring. People may experience a wide range of side effects; plus, certain conditions may affect the efficacy of the drugs, which need to be reported.
To ensure safe and efficient vaccine rollout, the Australian Department of Health Therapeutic Goods Administration (TGA) devised a COVID-19 vaccine safety monitoring plan.
Immunisation providers include aboriginal community controlled health Services, state and territory governments, primary health networks, General Practitioner-led Respiratory Clinics and, as of recently, community pharmacies and general practices. With the Australian government teaming up with so many different organisations, it’s important the vaccine monitoring plan follows uniform guidelines.
But what exactly needs to be monitored?
Adverse side effects
After the vaccine is administered, it’s possible the recipient will feel side effects. This is normal, and as long as they are mild, not particularly alarming. However, significant adverse effects, like allergic reactions or severe symptoms, should be reported because they are pertinent to vaccine safety monitoring.
Active surveillance of vaccine recipients after immunisation is not possible due to privacy concerns, so it’s essential that recipients understand the importance of reporting adverse vaccine events.
Reports on vaccine side effects help the TGA continue to develop its vaccine safety monitoring program. Every piece of recipient data allows the TGA to learn more about the vaccine’s effects, and this will make everyone safer. Vaccine recipients can report their adverse side effects by submitting a national adverse events following immunisation (AEFI) form.
‘Significant adverse effects, like allergic reactions or severe symptoms, should be reported because they are pertinent to vaccine safety monitoring.’
Patterns in AEFIs
When vaccine adverse event reporting occurs, the TGA compiles and organises data to find patterns and trends. This can lead to the TGA discovering concerns about the immunisation process that were previously undiscovered, which results in new research being conducted.
For example, if the adverse event reporting system reveals a side effect that was not seen in clinical trials, scientists will need to conduct research on underlying causes.
Additionally, if a certain group of people with unique conditions overwhelmingly experience side effects, recipient data will reveal a trend, and the group of people will be characterised as higher risk for the vaccine. This information will be distributed, enhancing public health and knowledge. Plus, scientists can begin working on a solution for these populations so they can still be protected from COVID-19.

Where temperature monitoring comes in
When recipient reports and safety monitoring reveal a high volume of ineffective vaccines or adverse side effects after vaccine administration from a particular site, experts may first direct their attention toward the storage units and cold chain.
The COVID-19 vaccine needs to be kept at very cold temperatures — ideally 5 degrees celsius, but anything between 2-8 degrees celsius is acceptable. However, it is known that the Pfizer vaccine must be stored at -70 degrees Celsius.
If the drug is exposed to a temperature outside of that range for more than 15 minutes, the incident is considered a cold chain breach, and the drug may have been rendered ineffective.
To ensure the drugs remain safe and effective, temperature monitoring equipment is a necessity. The cold chain begins as soon as the drug is produced and continues until it is administered. Once the vaccine reaches the facility, it only has a few days of shelf life before it expires. Monitoring temperature changes every step of the way is essential in accomplishing efficient and safe vaccine rollout.
‘Monitoring temperature changes every step of the way is essential in accomplishing efficient and safe vaccine rollout.’
Testo’s solutions
For a product as sensitive to changing temperatures as the COVID-19 vaccine, a reliable solution is necessary. That’s why Testo’s temperature measuring tools are the cutting-edge technology fit for a worldwide crisis like the pandemic.
Testo has many products to accomplish temperature monitoring throughout the cold chain. For one, the testo Saveris 2 T3, our WiFi-capable data logger system, is versatile, easy-to-use and highly reliable. It can be installed and operated through an internet browser, making it an efficient solution. Experts don’t need to waste precious time installing our products — their internet compatibility and user-friendly features make them perfect in situations where time is of the essence.
Transportation is one of the key hurdles in vaccine distribution. Keeping the vaccine at acceptable temperatures while frequently transferring it from one environment to another is a significant challenge. Luckily, the testo 184 is built for transportation purposes, even at temperatures as low as – 80 degrees Celsius for the Pfizer COVID-19 vaccine. It’s a data logger that monitors temperature changes throughout the entire cold chain. Since several people may be monitoring the temperatures of the storage units, readability is essential, and this product could not be more intuitive. With efficiency being of utmost importance, there is little room for error. The testo 184 allows for uninterrupted temperature monitoring to ensure little to no cold chain breaches.
Vaccine administration cannot be adequately monitored without active surveillance of the technology making it possible. If vaccine recipients report issues, there needs to be readily available data showing that the problem does not lie within the cold chain. Testo’s products ensure that the technology transporting and storing the vaccine is running exactly the way it should be, so there is no possibility of extraneous errors when a vaccine produces adverse effects.
Testo develops solutions that ensure safe and effective vaccine rollout. Contact us today for more information.







 Reduce cooking oil costs while ensuring quality
Reduce cooking oil costs while ensuring quality Expert knowledge on CO2 monitoring
Expert knowledge on CO2 monitoring Refrigeration knowledge - in 3 modules
Refrigeration knowledge - in 3 modules